Every day, scientists go into their laboratories and discover something new about the most recent coronavirus (COVID-19) outbreak. This explains why two months after the first ever case was reported, there are now three drugs expected to halt the proliferation of the virus in the body.
In all, more than 80 clinical trials have been launched to test coronavirus treatments including a stem-cell trial where scientists infuse patients with stem cell from menstrual blood.
It has been a battle to save lives and also, for both new and existing drugs to prove their worth.
As at February 21, nine vaccines and four drugs undergoing clinical trial have shown signs that they can handle the virus inside the human body. Of all the drugs, only three: Favilavir, Remdesivir and Chloroquine have been approved for use in patients infected with the virus.
Already, Favilavir and Remdesivir have been successfully used to treat coronavirus patients.
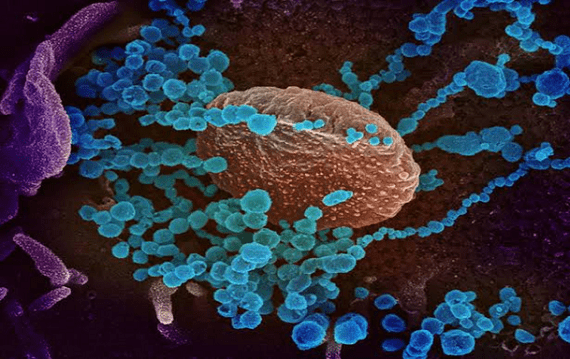
So how did the anti-malaria, Chloroquine come into the picture?
On February 4, Cell Research Journal published a study by researchers, majority from the Wuhan Institute of Virology, which declared remdesivir and chloroquine to be effective drugs in the treatment of the coronavirus.
Also, early data from trials being performed in China as noted by the Deputy Head, China National Centre for Biotechnology Development, Sun Yanrong revealed that chloroquine phosphate could help treat the new coronavirus disease, Covid-19.
Again, what does a defunct anti-malarial agent have to do with a virus as potent as the coronavirus?
The answer is, a lot.
Chloroquine has a broad spectrum anti-viral effect. Before now, there have been numerous HIV-drug trials involving chloroquine and its derivative, hydroxychloroquine. It has been discovered that they are able to inhibit glycosylation of viral receptors and induce production of non-infectious retrovirus particles in HIV-1.
According to the research, this forgotten anti-malarial agent is found to inhibit the virus as it enters and exits the body cells. It slows the progression of the infection and interferes with the glycosylation of the viral receptors in the host cell. It is able to achieve this in low micromolar concentrations.
This discovery about chloroquine is not unexpected. A research following the 2002 SARS coronavirus outbreak, a similar viral outbreak also in China, already demonstrated chloroquine’s potency against infections related to the coronavirus family.
The main problem regarding chloroquine is at what stage of the infection it can be effectively administered to someone sick with the coronavirus.
The second drug according to the Cell Research study is remdesivir. It is a nucleoside analog which shuts down transcription and synthesis of viral RNA.
Remdesivir was originally intended to treat the Ebola virus but failed during the human trials and replaced by more effective drugs.
However, with the new coronavirus, remdesivir and its manufacturers can make a timely comeback. In the United States, an infected patient was successfully treated with the drug and this has made the drug popular among researchers.
If remdesivir will eventually get approved, it is not going to be without some controversy. The research team at Wuhan Institute of Virology that facilitated the Cell Research study has filed a patent for the drug owned by the Gilead company in the United States. We don’t expect the manufacturers at Gilead to give up their intellectual property rights that easily.
Favilavir is the first ever coronavirus drug approved for use in patients. Before it was approved, a cruise ship of 2,666 passengers had been ravaged by the coronavirus and globally, 1863 deaths have been recorded.
Favilavir, an anti-viral product of a Chinese-based pharmaceutical company was originally developed to treat nose and throat inflammation caused by viruses.
The drug was cleared for use after it proved effective in a clinical trial of 70 patients with the virus. However, not much is known about the action of this antiviral drug because specific results of the clinical trial is yet to be released.
It is important to note that only Favilavir has been approved as a medicine for the new coronavirus. Remdesivir and chloroquine have only been cleared for human trials. They will be officially approved for use if they prove effective in reducing mortality rate among the human subjects infected with the virus.
Scientists have preferred to stick with pre-existing drugs in their search for the coronavirus treatment. It is a common thing during epidemics to use available drugs to control it as quickly as possible. This is because developing a new drug is painstakingly time consuming. It takes at least 10 years for a new drug to complete the journey from initial discovery to the market place.
The infection would have gone out of control and many lives lost before a drug is ready.
The coronavirus epidemic will not be the last of its kind and scientists know that.
For the future, scientists are building on available research works gathered from the two previous coronavirus outbreaks (SARS and MERS) as well as this current outbreak. They want to produce a one-in-all drug that would be able to combat the multiple strains of the coronavirus including the ones that haven’t surfaced yet.
We hope that we do not see a repeat of what happened during the SARS coronavirus outbreak, where the work starts and then stops as soon as the epidemic is gone.













6 Noble Virtues of the most perfect Wife
How becoming a good Housewife?Who is an effective wife? How for that father good wife? exactly what the Qualities of an ideal life partner? advantages of virtues she should have?
Wifely virtues are mentioned in Tulasi Ramayana (Sri Ramacharitamanasa) Aranya Kanda verse 4 as advice fond of Sita by Sage Atri’s wife Ansuya. according to the verse, ‘Devotion of metabolism, speech patterns and mind to her lord’s (husband’s) Feet is your only duty, sacred vow and penance of a woman’. The 6 Noble Virtues a housewife really need are summed up in the verses (with Neetisaram or Neeti Saara or Niti Shastra) awarded below.
Being a Good House WifeNeeti Saara is with individuals taking a collection of morals written by Telugu Poet Baddena aka Bhadra Bhupala who lived during the 13th century. Since this was written by an Indian bearing in mind the Hindu culture, It outlines the qualities an Indian bride likely has.
concept of ‘Karyeshu Dasi, Karaneshu Manthri; Bhojeshu Mata, Shayaneshu Rambha, Roopeshu lakshmi, Kshamayeshu Dharitri, Shat dharmayukta, Kuladharma Pathni’.
1) Karyeshu Dasi: Works becoming a servant
2) Karaneshu Mantri : suggests like a minister
3) Bhojeshu Mata: Feeds like a mother
4) Shayaneshu Ramba : Pleases in bed enjoy the heavenly beauty Rambha
5) Roopeshu Lakshmi : exceptional like Goddess Lakshmi
6) Kshmayeshu Dharitri : Having patience like Earth
7) Shat dharma yuktah: Woman who’s got this six virtues
8) Kula dharma Patni : incredibly good housewife (A married woman who is not employed away from home)
in brief, A good housewife have to have
Be like a servant in doing the chores of the property
Give sharp advice like a minister to her husband
Serve food to the husband as adoringly as a mother feeds her son
Like a courtesan in the bedroom
marvellous like Maha Lakshmi and
Have the forbearance of mother nature
As per the previous line of the shloka, A woman need to have all the above said qualities to be an ideal house wife. It also means that the lack of meet chinese girls even one quality makes a woman a failure as a good wife. Anyone getting these verses on Patni dharma (benefits of wife) Would know that is from a man. This age old notion of how the wife should be has entered the mind set of even modern men and woman because of such a shloka. Correct me if i am wrong. Is there a similar verse explaining the qualities of an ideal husband? I doubting! at the same time, The Hindu Dharma also has references about wife and husband relationship as ‘saha dharma chariNam’ which means sharing of dharma equally. It is also said that outcome or fruits of the chores would be shared equally. per Manu, ‘where women are honored, The Gods are satisfied; Where yet honored, All work turns to fruitless’.
in this type of 21st Century, The role of a wife has completely changed from what remained with us in the 1950s. The Modern Woman comes across as a strong, Independent and powerful individual who manages both home and office. Still her contribution and role in one’s life and family is often taken for granted. Her efforts are neither realized nor appreciated. thanks to the typical Indian male mindset. is really a popular qualities outlined in Neetisaaram are for that of a housewife (A wife who work from home), It is seen that most Indian Males expect their wives to match the role even if she is a working woman. I know a couple workers,who are your employees in a Bank. Both the husband and wife go to the Bank together and come back together. But the wife does all the work before going to bank and does all the work after coming back from work also with no help from husband at all because according to him running the household is her duty. irrespective of how free, intensifying, Educated and professional a woman is, Most Indian men see the household chores as a responsibility of a woman.
In this modern age, How far this six desirable traits or traits of an ideal wife are possible? no wonder that, Woman like me find most verses Male chauvinistic and patronizing. I find the hope of perfection (Goddess Lakshmi in Beauty and Rambha during sex) From women degrading. Do these traits apply regarding in this 21st century? A marriage is supposed to be an equal partnership between the wife and husband. Having said this I also agree that woman should thrive to make this happen 6 fold role to have a happy and successful marriage. I am a firm believer of ‘Treat your Husband identically you want yourself to be treated’. What you give is what you get in return. Give take delight in, Respect and care to your wife and you are sure to get it back from him. Even Niti Saara has precisely the same verse, ‘Do not do unto others what one wouldn’t like others to do unto oneself’.
Ballet trips and holidays paid for by patients who wouldn’t be able to enjoy them
She even bought the woman a 500 dress but the previous couple of words the woman said before she was bussed off to the theatre were smell. Covered up the cash withdrawals then told a senior carer to lie to the authorities.
The manager had handle of residents money at the Westwood Court Care Home in Winsford, Cheshire and withdrew the cash simply by which one had the most in the account.
Ward sister Kate Bithell described how regarding pounds of residents money would be handed to a carer and they were told it was for money. Of the residents would be chosen to pay extra for the staff, these Ms Bithell.
The one with the most money. It Charmdate.com was used for food and drink and any other outlays for the staff, She extra.
Went on holiday with several residents the year before and their money was used to pay for our expenses.
Would give me residents money and that could be for me and them. Would hand it to me and say get the dinner out of that. Hearing was told that two other users, Who both been inflicted by
Schizophrenia, Had a pooled total of 6,950 extracted from their accounts.
Ms Bithell told the hearing and seeing: Resident had cancers of the breast and at the end of her life.
was being bedbound, Frail and could not relay. Had no reason to spend handsome profit. needed 2,300 from her account between september 2012 and April 2013.
The only things the patient needed were toiletries they often bought from ASDA, replied Ms Bithell.
The same dying woman financed a trip to the ballet in Manchester even though she was unconscious on the performance.
Ms Bithell reported: Took them by minibus all the way up to Manchester.
On the trip they went for dinner at Pizza Express and had a McFlurry from McDonalds, The listening was told.
the patient, who were bedbound for 12 months, Was so frail that six nurses at the home said she ought not to go.
Had to hold her head plan my handbag and cardigan she was so slumped over, exclaimed Ms Bithell.
Was too ill to go the ballet and I told her Capner that, talked about Ms Bithell.
Ms Bithell told the hearing that the resident had whispered smell to her as they were getting ready to leave.
may possibly never taken her, She only agreed to be too poorly, She talked about.
Was ready to in bed in her room. Never asked for money and she wasn able to talk that she needed money she was so ill.
The NMC panel found that although woman could not speak she might have wanted to go to the ballet so misconduct in relation to the trip was not found proved.